A team of researchers from IBM Research Europe, Universidade de Santiago de Compostela and the University of Regensburg has changed the bonds between the atoms in a single molecule for the first time. In their paper published in the journal Science, the group describes their method and possible uses for it. Igor Alabugin and Chaowei Hu, have published a Perspective piece in the same journal issue outlining the work done by the team.
The current method for creating complex molecules or molecular devices, as Alagugin and Chaowei note, is generally quite challenging—they liken it to dumping a box of Legos in a washing machine and hoping that some useful connections are made. In this new effort, the research team has made such work considerably easier by using a scanning tunneling microscope (STM) to break the bonds in a molecule and then to customize the molecule by creating new bonds—a chemistry first.
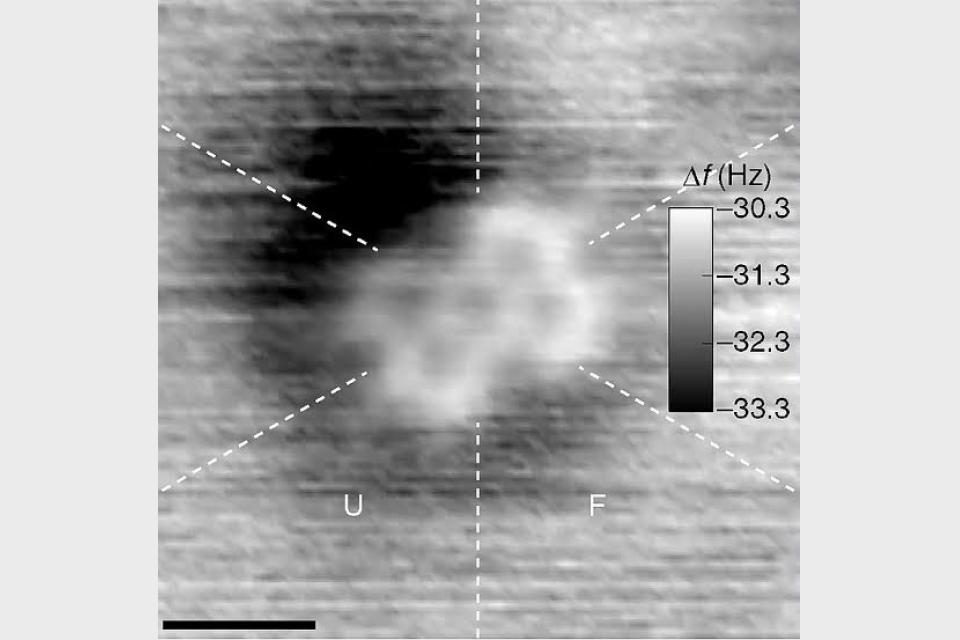
Schematic of the tip induced reactions. By voltage pulses from the tip of a scanning probe microscope, different molecular transformations are triggered selectively.
The work by the team involved placing a sample material into a scanning tunneling microscope and then using a very tiny amount of electricity to break specific bonds. More specifically, they began by pulling four chlorine atoms from the core of a tetracyclic to use as their starting molecule. They then moved the tip of the STM to a C-CI bond and then broke the bond with a jolt of electricity. Doing so to the other C-CI and C-C pairs resulted in the formation of a diradical, which left six electrons free for use in forming other bonds. In one test of creating a new molecule, the team then used the free electrons (and a dose of high voltage) to form diagonal C-C bonds, resulting in the creation of a bent alkyne. In another example, they applied a dose of low voltage to create a cyclobutadiene ring.
The researchers note that their work was made possible by the development of ultrahigh precision tunneling technology developed by a team headed by Gerd Binnig and Heinrich Rohrer, both with IBM's laboratory in Zurich. They suggest their technique could be used to better understand redox chemistry and to create new kinds of molecules.